In ISO/IEC 17025, "General Requirements for Competence of Calibration and Testing Laboratories," it is clearly stated that test reports issued by laboratories should include indications of uncertainty in the evaluation of calibration or test results. Therefore, the evaluation of the uncertainty of measurement results becomes a test. The important content of the laboratory. In this paper, the uncertainty of the test results of protein testing items in foods was evaluated.
1 Inspection method
Principle; Nitrogen-containing organic compounds, together with sulfuric acid, are heated and digested to decompose protein. The decomposed ammonia is combined with sulfuric acid to produce ammonium sulfate, then alkalized and distilled to make ammonia free. After being absorbed by boric acid, it is titrated with hydrochloric acid standard solution according to acid. Consumption is multiplied by the conversion factor as protein content.
Build a mathematical model
1.1 Relative Standard Uncertainty of Hydrochloric Acid Standard Solution u(CHCL)/CHCL Analysis The uncertainty of hydrochloric acid standard solution comes from three aspects: First, the purity of reference inorganic sodium carbonate used for calibration is 100% ± 0.05%, which is evenly distributed. The standard deviation was 0.0005/√ 3=0.00029, u(p)/p=2.9 × 10-4. Second, the variability of the reference weighing, variability measured within 50g is measured by 10 repeated measurements, the standard deviation is 0.05mg, and the uncertainty resulting from balance calibration is ± 0.1mg given by inspection certificate. When the confidence probability is 95% (k=1.96), the standard deviation is 0.1/1.96=0.051, that is, u (m') / m' = 5. 4 × 1 0 -4. Third, the uncertainty of the 50 ml burette is ± 0.04 mL given by the test certificate. The standard deviation in terms of uniform distribution is 0.04/√ 3 = 0.023 mL. The variability of the liquid-filled burette scale is repeated six times. Statistical, standard deviation 0.012mL, volumetric uncertainty caused by different temperature between the burette and the solution temperature correction, assuming a difference of 2 °C, water volume expansion coefficient of 2.1 × 10-4 / °C, 95% confidence probability (k = 1.96) The time volume change interval was ± 50 × 2 × 2.1 × 10-4 = ± 0.021 mL, and the conversion standard deviation was 0.021/1.96 = 0.011. u(V50)/V50=5.6 × 10-4. The relative standard uncertainty of the hydrochloric acid standard solution obtained from the above three items is:

1.2 Analysis of the standard uncertainty of the sample weighing u(m) The analytical weighing uncertainty comes from two aspects. First, the variability of the weighing is within 50 g, and the variability of the weighing is measured by 10 repeated measurements. The standard deviation is 0.05 mg. Second, the uncertainty resulting from balance calibration is ±0.1mg given by inspection certificate. When the confidence probability is 95% (k=196), the standard deviation is: 0.1/1.96=0.051. The weighing uncertainty is:
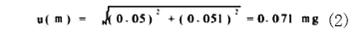
1.3 Analysis of the Standard Uncertainty u(V100) of 100mL Digestion Bottle Volume
The uncertainty of the 100mL digester bottle comes from three aspects. First, the uncertainty of the digester volume is given as ± 0.12 mL according to the inspection certificate, and the standard deviation is 0.12/ √ 3 = 0.069 mL according to the uniform distribution. Second, the variability of the liquid-filled scale to the digester was measured by 6 replicates with a standard deviation of 0.019 mL. Third, the volumetric uncertainty caused by the difference between the temperature of the digestion bottle and the solution and the calibration temperature is assumed to be 2°C, and the volume expansion coefficient for water is 2.1×10 −4 /° C., then the 95% confidence probability (k=1.96). The time volume change range is ± 100 × 2 × 2.1 × 10-4 = ± 0.042 mL, and the standard deviation is 0.042/1.96 = 0.021 mL. The uncertainty of these three items is:
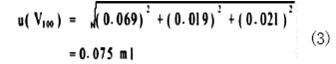
Analysis of the Standard Uncertainty u(V20) of a 20mL Pipette
The standard uncertainty of the 20 mL pipette is: First, the uncertainty of the pipette volume is given as ±0.03 mL according to the inspection certificate, and the standard deviation is 0.03/√ 3=0.017 mL. Second, full of liquid to pipette scale variability, by repeating the determination of statistics six times, the standard deviation of 0.012mL; third, the volumetric uncertainty of the pipette and solution temperature and the temperature difference between the calibration, assumption With a difference of 2°C and a volumetric expansion coefficient of water of 2.1 × 10-4/°C, the volume change interval for the 95% confidence probability (k = 1.96) is ± 20 × 2 × 2.1 × 10-4 = ± 0.008 mL. The standard deviation is 0.008/1.96 = 0.004 mL. The uncertainty of the above three combinations is:
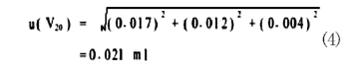
1.5 Analysis of Uncertainty u(V10) of Depletion of Hydrochloric Acid Standard Solution from Reagent Blank (V1-V0) Samples
The titration of the sample and reagent blank with the hydrochloric acid standard solution was performed in a 10 mL burette. The standard uncertainty of the 10mL buret is: First, the uncertainty of the burette volume is given as ±0.02mL according to the verification certificate, and the standard deviation is 0.02/√3=0.012mL according to the uniform distribution. Second, the variability of the liquid-filled burette scale was determined by repeating the determination six times with a standard deviation of 0.011 mL. Third, the uncertainty of the volume change caused by the difference between the temperature of the burette and the solution and the calibration temperature, assuming a difference of 2°C, and a water volume expansion coefficient of 2.1 × 10-4/°C, a 95% confidence probability (k=1.96) The time volume change interval is ± 10 × 2 × 2.1 × 10-4 = 0.004 mL, and the standard deviation is 0.004/1.96 = 0.002 mL. The uncertainty of the above three combinations is:

The sample (soybean meal) had a protein content of N=44.32 g/100 g, and u(N)=44.32×0.0023=0.10 g/100 g. Of course, there is also a very simple method for determining the protein content of soybeans, that is, directly measuring with an instrument. For example, the crude protein analyzer is an instrument for measuring protein, and an electric steam generator is used, which can continuously carry out trace and constant distillation. Contains 8-well digital digester.
2 Extended uncertainty analysis
Taking the inclusion factor k = 2 (95% confidence probability), U(N) = 2 × u(N) = 2 × 0.10 = 0.20g/100g.
3 The final result is expressed
N=N±U(N)=(44.32±0.20)g/100g.
HVOF PTA Powder,Metal Spray Powder,Flame Spray Powder,Metal Alloy Powder
Luoyang Golden Egret Geotools Co., Ltd , https://www.xtccarbide.com