[ Instrument R & D of Instrument Network ] Nitric oxide (NOC) is a highly conserved signaling molecule that participates in regulating many biological processes. NO is one of the main ways of exerting biological functions through S-nitrosylation of target protein-specific cysteine ​​residues. Unlike post-translational modifications of other proteins, nitrosation modification was considered to be a non-enzymatic reaction, its specificity mainly depends on the local concentration of NO and the structure of the target protein. Recent studies have discovered a new mechanism of selectivity for S-nitroso modification, that is, a type of protein can transfer the NO group it carries to another protein, resulting in the latter's nitroso modification This process is called transnitrosylation, and the protein that mediates transnitrosylation is called transnitrosylase. At present, several transnitrosylases with very different structures have been found in animals and E. coli. In plants, no transnitrosase has been found.
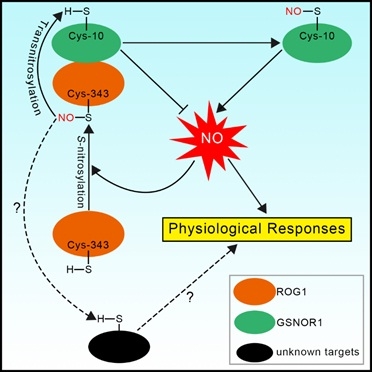
The Zuo Jianru research group and collaborators of the State Key Laboratory of Plant Genomics, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences recently discovered plant-specific transnitrosases. The main biologically active form of NO is S-nitrosoglutathione (GSNO), which can be irreversibly decomposed by highly conserved GSNO reductase (GSNOR). Therefore, GSNOR is the main control factor that regulates NO homeostasis. The mutation of gsnor in different species leads to increased NO levels and various serious defects. Through genetic screening, the research team obtained the repressor of gsnor1 mutant of the Arabidopsis gsnor1 mutant. In addition to inhibiting the phenotype of the gsnor1 mutant, the sensitivity of the rog1 mutant to NO was significantly reduced, indicating that ROG1 is an important component in regulating the NO signaling pathway. Molecular genetics and biochemical studies have found that ROG1 encodes a transnitrosylase, one of its substrates is GSNOR1 itself. ROG1 mediates the nitrosation modification of GSNOR1 which causes it to be degraded through the autophagy pathway, thereby forming a positive feedback loop to regulate the NO signaling pathway.
Surprisingly, ROG1 is catalase CAT3 (catalase 3). ROG1 transnitrosase has only very low catalase activity; in contrast, its homologous protein CAT2 has high catalase activity but very low transnitrosase activity. A major factor determining the specificity of ROG1 and CAT2 enzyme activities is their specific Cys-343 residues and Thr-343 residues. After replacing Cys-343 of ROG1 with Thr, its transnitrosase activity was significantly reduced, while catalase activity increased; conversely, after replacing Thr-343 of CAT2 with Cys, its transnitroso Catalase activity increased, while catalase activity decreased. Similar enzyme activity analysis of rice ROG1-like protein (OsCATA) and CAT2-like protein (OsCATC) reached similar conclusions, indicating that this is a highly conserved mechanism in plants. This study discovered a new mechanism for regulating plant-specific selective S-nitroso modification.
The above research was carried out in collaboration with Zuo Jianru Research Group, Zhou Jianmin Research Group, Li Jiayang Research Group, and Song Chunpeng Research Group of Henan University. Related results were published online on April 23 in Developmental Cell (DOI: 10.1016 / j.devcel.2020.03.020). Chen Lichao, a postdoctoral fellow in the Zuo Jianru research group, is the first author of the paper, and Zuo Jianru is the corresponding author. The research was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the State Key Laboratory of Plant Genomics.
Jining Xunda Pipe Coating Material Co.,Ltd , https://www.alta-altene.com